Potassium Dichromate Colour Change
Na 2 SO 3 H 2 SO 4 Na 2 SO 4 H 2 O SO 2 The gas turns potassium dichromate paper acidified with dil. Materials Required Apparatus Required weighing bottle weight box volumetric flask conical flask.

Ethanol Oxidation Stock Image C036 3138 Science Photo Library
This colour change of orange to yellow and yellow to orange can be explained on the concept of equilibrium of the two ions.

. H 2 SO 4 green. Search all type Industrial Raw Materials its Sellers in India Browse by Industry Category Send Inquiry Get Multiple Sellers Responses List Raw Material Products PlasticsPolymer PharmaceuticalsDrug Chemicals Paints PesticidesInsecticide MetalsSteel OreMineral YarnFibre RubberElastomer Construction CeramicsGlass Fertilizers. 4K 2 Cr 2 O 7 4K 2 CrO 4 2Cr 2 O 3 3O 2.
When the solution of K 2 Cr 2 O 7 reacts with an alkali ionic salt a yellow solution is obtained because of the potassium. K 2 Cr. You should check the result as soon as the potassium dichromateVI solution turns green - if you leave it too long the Schiffs reagent might start to change colour in the secondary alcohol case as well.
Introducing heat to K 2 Cr 2 O 7 decomposes it into potassium chromate K 2 CrO 4 and produces O 2 gas. Enter the email address you signed up with and well email you a reset link. H 2 SO 4 SO 2 gas is evolved which is suffocating with the smell of bur ning sulphur.
2KMnO 4 3H 2 SO 4 K 2 SO 4 2MnSO 4 3H 2 O 5O Or MnO 4 8H 5e Mn 2 4H 2 O. Iii Change in state of substance. In this titration the potassium permanganate is used as an oxidizing agent.
Reaction to form hydrogen chromate ions or dichromate ions affecting the accuracy of the end point. 307 Triple only use bond energies to calculate the enthalpy change during a chemical reaction. The Mohr titration is sensitive to the presence of both chloride and bromide ions in solution and.
In this reaction. A common method for Chemical Oxygen Demand analysis involves using a strong oxidizing chemical potassium dichromate Cr 2 O 7 2- to oxidize the organic matter in solution to carbon dioxide and water under acidic conditions. The sodium chromate is dissolved in water and this solutionis acidified with sulfuric acid to produce the less soluble sodiumdichromate.
Great Deals Fast Shipping. The sodium dichromate is filtered out and reduced with carbon to produce chromiumIII oxide sodium carbonateand carbon monoxide. Cr 2 O 7 2- H 2 O 2CrO 4 2- 2H Orange-red Yellow.
Chemical Properties of Potassium Dichromate. The chemical reaction between sulphur dioxide gas and acidified potassium dichromate solution is characterized by a change in colour from orange to green. From the cystine amino acid which act as reducing agent and can reduce hexavalent chromium of potassium dichromate to trivalent form.
The appearance of the endpoint can be detected by the colour change of KMnO 4 from colourless to light pink. If the potassium dichromate solution changes colour from orange to green then an oxidation reaction has taken place with a primary or secondary alcohol. The chromiumIII oxide is then reduced to chromium with aluminum metal.
If no colour change is observed then the alcohol was a tertiary alcohol. Here is the equation. Aim To prepare M20 solution of Mohrs salt and.
The trivalent chromium forms the complex with the fibre and dye. Ad Choose From 300000 Chemicals From Certified Manufacturers. 410 know the trend in colour boiling point and viscosity of the main fractions.
Test for Sulphite ion SO2 3 a On treating sulphite with warm dil. The type of mordant used can change the colour of both the dye-plus-mordant solution and influence the shade of the final product. The color of chemicals is a physical property of chemicals that in most cases comes from the excitation of electrons due to an absorption of energy performed by the chemical.
Sodium nitroprusside Complex of Purple colour 3. Because of the colour change to the acidified potassium dichromateVI solution you must therefore have a secondary alcohol. Mohrs salt Titration with Potassium Dichromate.
It is a good idea to first carry out a rough titration in order to become familiar with the colour change at the end point. Iodimetric and Iodometric Titrations. What is seen by the eye is not the color absorbed but the complementary color from the removal of the absorbed wavelengthsThis spectral perspective was first noted in atomic spectroscopy.
A primary alcohol will form an aldehyde and a secondary alcohol will form a ketone. ChemDirect Powers The Best Chemical Buying Selling Experience Online. The combustion reaction of candle wax is characterised by a change in state from solid to liquid and gas because the wax is a solid water formed by the.
Butanol is oxidised by sodium dichromate Na 2 Cr 2 O 7 acidified in dilute sulphuric acid to form the aldehyde butanal. In the potassium dichromate solution Cr 2 O 7 2-ions in orange colour are in equilibrium with CrO 4 2-ions in yellow colour. The oxidation of the alcohol to an aldehyde is indicated by the colour change of the dichromate solution as it is reduced from the orange colour of Cr 2 O 7 2 to the green of chromiumIII ions Cr 3.
Complete combustion reaction with oxygen in the air to form ethanoic acid microbial oxidation heating with potassium dichromateVI in dilute sulfuric. It is maintained with the use of dilute sulphuric acid.

The Colour Change Of Acidified Potassium Dichromate The Real Chemist Youtube
What Is The Precipitate In Potassium Dichromate Sulfuric Acid And Potassium Iodide Quora

Organic Analysis Flashcards Quizlet
Why Does The Colour Change While Potassium Dichromate And Ethyl Alcohol Is Added Quora
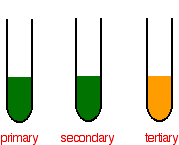
14 7 Determining Alcohol Classifications In The Lab Alternate Reactions Chemistry Libretexts

Use Of Acidified Potassium Dichromate To Distinguish Between Alehydes And Ketones Youtube
0 Response to "Potassium Dichromate Colour Change"
Post a Comment